Holt Biology Energy in Living Systems Directed Reading
Cellular Respiration
33 Free energy in Living Systems
Learning Objectives
By the end of this section, you will be able to do the following:
- Discuss the importance of electrons in the transfer of free energy in living systems
- Explain how ATP is used past cells every bit an energy source
Energy production within a prison cell involves many coordinated chemical pathways. Most of these pathways are combinations of oxidation and reduction reactions, which occur at the same fourth dimension. An oxidation reaction strips an electron from an atom in a compound, and the add-on of this electron to another chemical compound is a reduction reaction. Because oxidation and reduction commonly occur together, these pairs of reactions are called oxidation reduction reactions, or redox reactions.
Electrons and Energy
The removal of an electron from a molecule (oxidizing it), results in a decrease in potential energy in the oxidized chemical compound. The electron (sometimes every bit part of a hydrogen atom) does not remain unbonded, however, in the cytoplasm of a cell. Rather, the electron is shifted to a second chemical compound, reducing the second chemical compound. The shift of an electron from one compound to another removes some potential energy from the first compound (the oxidized compound) and increases the potential energy of the second compound (the reduced chemical compound). The transfer of electrons between molecules is important considering most of the free energy stored in atoms and used to fuel cell functions is in the form of high-energy electrons. The transfer of energy in the course of loftier-energy electrons allows the prison cell to transfer and use free energy in an incremental mode—in small packages rather than in a single, destructive burst. This chapter focuses on the extraction of energy from food; you will see that as you rail the path of the transfers, yous are tracking the path of electrons moving through metabolic pathways.
Electron Carriers
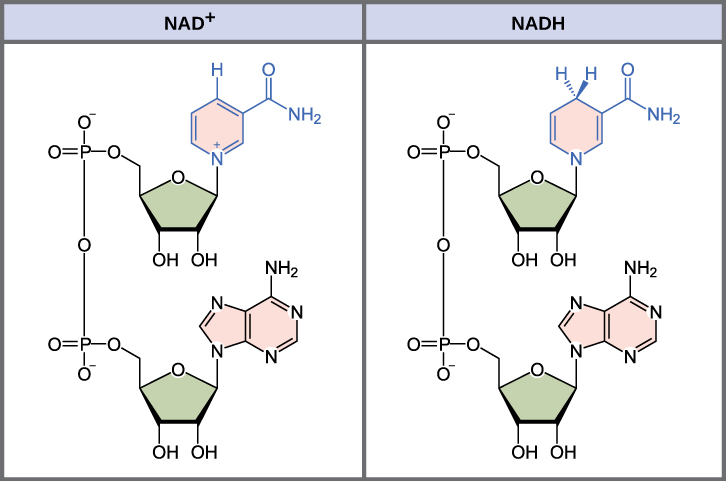
In living systems, a small class of compounds functions every bit electron shuttles: they demark and carry high-energy electrons betwixt compounds in biochemical pathways. The master electron carriers nosotros will consider are derived from the B vitamin group and are derivatives of nucleotides. These compounds can be easily reduced (that is, they have electrons) or oxidized (they lose electrons). Nicotinamide adenine dinucleotide (NAD) ((Figure)) is derived from vitamin Biii, niacin. NAD+ is the oxidized form of the molecule; NADH is the reduced form of the molecule after it has accepted ii electrons and a proton (which together are the equivalent of a hydrogen atom with an actress electron). Note that if a compound has an "H" on it, it is generally reduced (e.g., NADH is the reduced form of NAD).
NAD+ can accept electrons from an organic molecule according to the full general equation:
[latex]\begin{array}{c}\text{RH}\\ \begin{array}{c}\text{Reducing }\\ \text{agent}\cease{array}\end{array}\text{ + }\begin{array}{c}{\text{NAD}}^{+}\\ \begin{assortment}{c}\text{Oxidizing}\\ \text{agent}\terminate{array}\end{array}\to \text{ }\begin{array}{c}\text{NADH}\\ \text{Reduced}\end{array}\text{ +}\begin{assortment}{c}\text{R}\\ \text{Oxidized}\end{assortment}[/latex]
When electrons are added to a compound, information technology is reduced. A compound that reduces another is called a reducing amanuensis. In the above equation, RH is a reducing amanuensis, and NAD+ is reduced to NADH. When electrons are removed from a compound, it is oxidized. A compound that oxidizes another is called an oxidizing agent. In the to a higher place equation, NAD+ is an oxidizing agent, and RH is oxidized to R.
Similarly, flavin adenine dinucleotide (FAD+) is derived from vitamin B2, too called riboflavin. Its reduced course is FADHii. A second variation of NAD, NADP, contains an extra phosphate group. Both NAD+ and FAD+ are extensively used in energy extraction from sugars, and NADP plays an important role in anabolic reactions and photosynthesis in plants.
The oxidized form of the electron carrier (NAD+) is shown on the left, and the reduced class (NADH) is shown on the right. The nitrogenous base in NADH has one more hydrogen ion and 2 more electrons than in NAD+.
ATP in Living Systems
A living cell cannot store meaning amounts of energy. Backlog gratuitous free energy would result in an increase of heat in the cell, which would result in excessive thermal motion that could damage so destroy the cell. Rather, a cell must exist able to handle that free energy in a mode that enables the jail cell to store free energy safely and release it for utilise only as needed. Living cells accomplish this past using the chemical compound adenosine triphosphate (ATP). ATP is often chosen the "free energy currency" of the prison cell, and, similar currency, this versatile compound can exist used to fill any energy need of the cell. How? It functions similarly to a rechargeable battery.
When ATP is cleaved down, usually past the removal of its last phosphate group, free energy is released. The energy is used to practice work by the prison cell, usually when the released phosphate binds to another molecule, thereby activating it. For example, in the mechanical work of muscle contraction, ATP supplies the energy to movement the contractile muscle proteins. Recollect the agile transport piece of work of the sodium-potassium pump in cell membranes. ATP alters the construction of the integral poly peptide that functions as the pump, changing its affinity for sodium and potassium. In this way, the cell performs work, pumping ions confronting their electrochemical gradients.
ATP Structure and Function
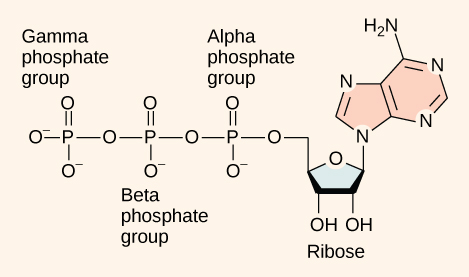
At the heart of ATP is a molecule of adenosine monophosphate (AMP), which is composed of an adenine molecule bonded to a ribose molecule and to a single phosphate group ((Figure)). Ribose is a five-carbon sugar found in RNA, and AMP is 1 of the nucleotides in RNA. The add-on of a second phosphate grouping to this cadre molecule results in the germination of adenosine diphosphate (ADP); the addition of a third phosphate group forms adenosine triphosphate (ATP).
ATP (adenosine triphosphate) has three phosphate groups that can exist removed by hydrolysis (add-on of H2O) to course ADP (adenosine diphosphate) or AMP (adenosine monophosphate). The negative charges on the phosphate group naturally repel each other, requiring energy to bond them together and releasing energy when these bonds are broken.
The addition of a phosphate group to a molecule requires energy. Phosphate groups are negatively charged and thus repel 1 some other when they are arranged in series, as they are in ADP and ATP. This repulsion makes the ADP and ATP molecules inherently unstable. The release of one or two phosphate groups from ATP, a process called dephosphorylation, releases free energy.
Free energy from ATP
Hydrolysis is the procedure of breaking complex macromolecules apart. During hydrolysis, h2o is carve up, or lysed, and the resulting hydrogen atom (H+) and a hydroxyl group (OH–), or hydroxide, are added to the larger molecule. The hydrolysis of ATP produces ADP, together with an inorganic phosphate ion (Pi), and the release of energy. To carry out life processes, ATP is continuously broken downward into ADP, and like a rechargeable battery, ADP is continuously regenerated into ATP by the reattachment of a 3rd phosphate group. Water, which was broken downwards into its hydrogen cantlet and hydroxyl group (hydroxide) during ATP hydrolysis, is regenerated when a tertiary phosphate is added to the ADP molecule, reforming ATP.
Plainly, free energy must be infused into the system to regenerate ATP. Where does this energy come from? In nearly every living thing on Earth, the energy comes from the metabolism of glucose, fructose, or galactose, all isomers with the chemical formula C6H12O6 only unlike molecular configurations. In this style, ATP is a direct link between the limited set of exergonic pathways of glucose catabolism and the multitude of endergonic pathways that power living cells.
Phosphorylation
Recall that, in some chemic reactions, enzymes may demark to several substrates that react with each other on the enzyme, forming an intermediate complex. An intermediate complex is a temporary structure, and it allows one of the substrates (such as ATP) and reactants to more than readily react with each other; in reactions involving ATP, ATP is one of the substrates and ADP is a production. During an endergonic chemical reaction, ATP forms an intermediate complex with the substrate and enzyme in the reaction. This intermediate circuitous allows the ATP to transfer its third phosphate group, with its free energy, to the substrate, a process called phosphorylation. Phosphorylation refers to the addition of the phosphate (~P). This is illustrated by the post-obit generic reaction, in which A and B stand for two dissimilar substrates:
[latex]\text{A + enzyme + ATP}\to \text{ }\left[\text{A }-\text{ enzyme }-\text{ }\sim \text{P}\right]\text{ }\to \text{ B + enzyme + ADP + phosphate ion}[/latex]
When the intermediate complex breaks apart, the energy is used to modify the substrate and convert it into a product of the reaction. The ADP molecule and a free phosphate ion are released into the medium and are available for recycling through cell metabolism.
Substrate Phosphorylation
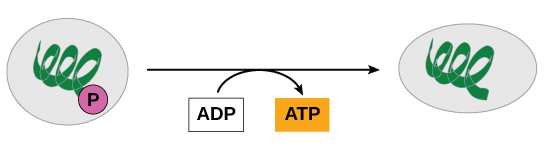
ATP is generated through two mechanisms during the breakdown of glucose. A few ATP molecules are generated (that is, regenerated from ADP) every bit a directly result of the chemical reactions that occur in the catabolic pathways. A phosphate group is removed from an intermediate reactant in the pathway, and the free energy of the reaction is used to add the 3rd phosphate to an available ADP molecule, producing ATP ((Figure)). This very direct method of phosphorylation is chosen substrate-level phosphorylation.
In phosphorylation reactions, the gamma (tertiary) phosphate of ATP is attached to a protein.
Oxidative Phosphorylation
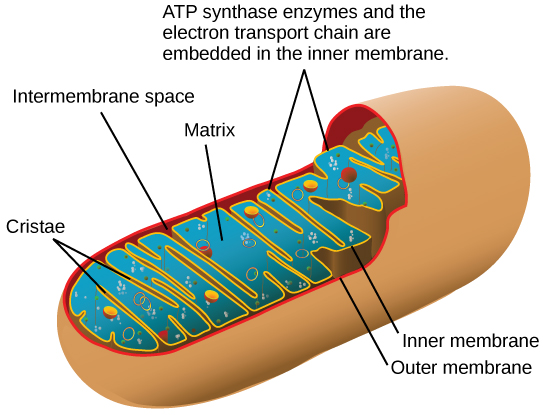
Most of the ATP generated during glucose catabolism, nonetheless, is derived from a much more complex process, chemiosmosis, which takes place in mitochondria ((Figure)) within a eukaryotic cell or the plasma membrane of a prokaryotic prison cell. Chemiosmosis, a process of ATP product in cellular metabolism, is used to generate 90 percentage of the ATP made during glucose catabolism and is likewise the method used in the calorie-free reactions of photosynthesis to harness the energy of sunlight. The production of ATP using the process of chemiosmosis is chosen oxidative phosphorylation because of the involvement of oxygen in the process.
In eukaryotes, oxidative phosphorylation takes place in mitochondria. In prokaryotes, this process takes place in the plasma membrane. (Credit: modification of piece of work past Mariana Ruiz Villareal)
Career Connections
Mitochondrial Affliction PhysicianWhat happens when the critical reactions of cellular respiration practise non proceed correctly? This may happen in mitochondrial diseases, which are genetic disorders of metabolism. Mitochondrial disorders can ascend from mutations in nuclear or mitochondrial Dna, and they result in the production of less free energy than is normal in body cells. In type ii diabetes, for instance, the oxidation efficiency of NADH is reduced, impacting oxidative phosphorylation just non the other steps of respiration. Symptoms of mitochondrial diseases can include muscle weakness, lack of coordination, stroke-like episodes, and loss of vision and hearing. Most afflicted people are diagnosed in childhood, although there are some adult-onset diseases. Identifying and treating mitochondrial disorders is a specialized medical field. The educational preparation for this profession requires a college pedagogy, followed by medical schoolhouse with a specialization in medical genetics. Medical geneticists tin be board certified by the American Board of Medical Genetics and keep to become associated with professional person organizations devoted to the study of mitochondrial diseases, such every bit the Mitochondrial Medicine Society and the Society for Inherited Metabolic Disorders.
Section Summary
ATP functions as the energy currency for cells. It allows the cell to shop free energy briefly and transport it within the cell to back up endergonic chemical reactions. The structure of ATP is that of an RNA nucleotide with three phosphates attached. As ATP is used for energy, a phosphate group or 2 are discrete, and either ADP or AMP is produced. Energy derived from glucose catabolism is used to convert ADP into ATP. When ATP is used in a reaction, the third phosphate is temporarily attached to a substrate in a procedure chosen phosphorylation. The two processes of ATP regeneration that are used in conjunction with glucose catabolism are substrate-level phosphorylation and oxidative phosphorylation through the process of chemiosmosis.
Review Questions
The energy currency used by cells is ________.
- ATP
- ADP
- AMP
- adenosine
A
A reducing chemical reaction ________.
- reduces the chemical compound to a simpler form
- adds an electron to the substrate
- removes a hydrogen atom from the substrate
- is a catabolic reaction
B
Disquisitional Thinking Questions
Why is it beneficial for cells to utilize ATP rather than energy directly from the bonds of carbohydrates? What are the greatest drawbacks to harnessing energy direct from the bonds of several unlike compounds?
ATP provides the cell with a way to handle free energy in an efficient manner. The molecule can be charged, stored, and used as needed. Moreover, the energy from hydrolyzing ATP is delivered as a consistent amount. Harvesting free energy from the bonds of several different compounds would result in free energy deliveries of unlike quantities.
Glossary
- chemiosmosis
- procedure in which at that place is a production of adenosine triphosphate (ATP) in cellular metabolism by the interest of a proton gradient beyond a membrane
- dephosphorylation
- removal of a phosphate group from a molecule
- oxidative phosphorylation
- product of ATP using the process of chemiosmosis in the presence of oxygen
- phosphorylation
- improver of a loftier-energy phosphate to a chemical compound, usually a metabolic intermediate, a protein, or ADP
- redox reaction
- chemical reaction that consists of the coupling of an oxidation reaction and a reduction reaction
- substrate-level phosphorylation
- production of ATP from ADP using the excess energy from a chemical reaction and a phosphate group from a reactant
Source: https://opentextbc.ca/biology2eopenstax/chapter/energy-in-living-systems/
Post a Comment for "Holt Biology Energy in Living Systems Directed Reading"